The International Coordinating Group (ICG) on Vaccine Provision was established in 1997, following major outbreaks of meningitis in Africa, as a mechanism to manage and coordinate the provision of emergency vaccine supplies and antibiotics to countries during major outbreaks.
Although the occurrence of outbreaks is often unpredictable, further disease spread can be controlled by timely vaccination. People living in vulnerable settings are at higher risk of infectious disease outbreaks. Unfortunately, the risk is further increased because vaccines are often in limited supply and not readily available in the amounts needed to respond to emergencies in vulnerable settings. Ensuring that limited vaccines supplies are prioritized where they are most needed is crucial.
Contact
ICG mandate:
- The core mandate of the ICG is to make available and ensure equitable access to licensed vaccines for cholera, meningitis, yellow fever, and Ebola virus disease during outbreaks.
- The ICG mechanism seeks to ensure timely and targeted deployment of vaccines so that they can be used effectively in emergency settings where they are most needed.
- The ICG manages the global emergency vaccine stockpiles and, working with partners, donors, and manufacturers, determines their size and composition with the goal of ensuring that adequate stocks of emergency supplies are accessible for emergency response.
Vaccine stockpiles
ICGs have been established to provide access to vaccines for the following diseases:
Meningitis - The ICG on vaccine provision for epidemic meningitis control was established in January 1997. Since its establishment until October 2022, almost 64 million doses of vaccines were shipped to 21 countries for outbreak response. For 2023, the ICG members recommended that the ICG meningococcal vaccine stockpile should be 4 million doses of C-containing vaccine, of which at least 3 million should be CW-containing.
Yellow fever – The ICG mechanism for yellow fever vaccines was established in 2001. Since then until October 2022, approximately 99 million doses of yellow fever vaccine were released and shipped to 20 countries facing outbreaks. A stockpile of 6 million doses available at all times is reserved for outbreak response since May 2016.
Cholera - Since 2013, the ICG for cholera vaccines manages the emergency stockpile of oral cholera vaccine (OCV) which was created as an additional tool to help control cholera epidemics. Since then, until October 2022, almost 73 million doses of OCV were shipped to 23 countries for emergency response. A stockpile of 5 million doses available at all times is reserved for outbreak response since June 2022.
Ebola - The ICG manages the emergency stockpile of Ebola vaccine since January 2021, which was created as an additional tool to control Ebola outbreaks. As Ebola outbreaks are relatively rare and unpredictable in nature, and due to limited vaccine quantities, the current Ervebo vaccine is reserved for outbreak response to protect people at the highest risk of contracting Ebola – including health care and frontline workers responding to the outbreak – under a ring vaccination strategy. Since the establishment of ICG Ebola mechanism in 2021 until October 2022, a total of 7,370 doses of Ervebo vaccine have been allocated to respond to Ebola outbreaks in DRC. A global stockpile of 500,000 doses of Ebola vaccine has been constitute and will be available at all times for outbreak response.
Partners
The ICG is made up of four member agencies:
- International Federation of the Red Cross and Red Crescent Societies (IFRC) - Has strong country presence for community health promotion, local social and resource mobilization and provides support to states during disasters and epidemics.
- Médecins sans Frontières (MSF) - An independent, field-based NGO that provides health care to vulnerable populations in emergency settings.
- United Nations Children’s Fund (UNICEF) - Conducts wide scale vaccine procurement and shipment, and provides technical support on campaign planning and implementation in country focusing specially on social mobilization and cold chain.
- World Health Organization (WHO) - Provides global public health advice and technical support to countries. During outbreaks, WHO focuses on vaccine stockpile management, surveillance, preparedness and response to disease outbreaks. WHO also functions as Secretariat of the ICG
Vaccine manufacturers, vaccine equipment providers and financial donor institutions are also engaged in the ICG operations.
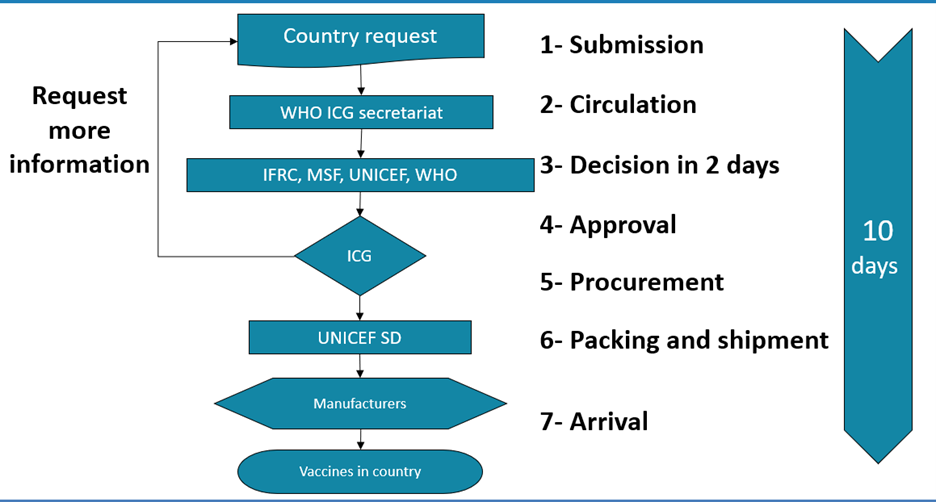
ICG process to release vaccines from the emergency stockpiles
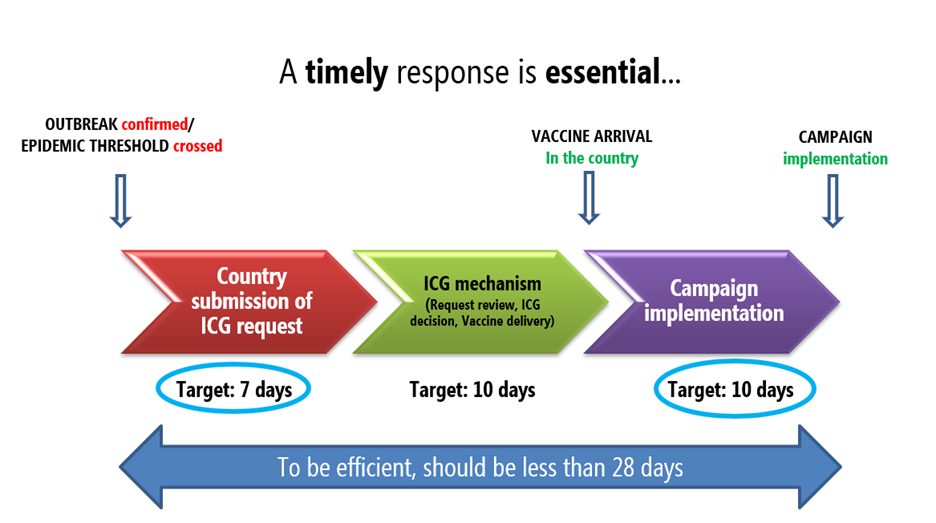
Vaccine security stocks can be accessed by ANY country facing an epidemic ANYWHERE in the world, as long as the country’s request fulfills ICG’s criteria for release of vaccine stocks. As a first step, a country must complete and submit a request to the ICG Secretariat using the standard application form. The annexes and other relevant documents should be submitted with the application as per the checklist in the ICG request form. Countries are encouraged to submit the request to the ICG Secretariat within 7 days after confirmation of the outbreak.
The ICG secretariat at WHO then circulates the request to the ICG members from the four agencies within 1 day after reception and review of country’s submission. Additional information can be requested to the country. Following a rapid consultation and evaluation process, the ICG members decide by consensus on the release of vaccines and other supplies to the requesting country within 2 working days, once all necessary information has been provided. If approved, UNICEF Supply Division organizes the delivery of vaccines to the country within 7 days.
Requests are evaluated taking into account the epidemiological situation, vaccination strategy, vaccine availability in the emergency stockpile (https://www.unicef.org/supply/emergency-vaccine-stockpile), pre-existing stocks in the country and operational aspects of the epidemic response.
Managing, procuring and purchasing vaccine supplies
The ICG ensures that contingency stocks of vaccines are available to immediately respond to disease outbreaks.
The emergency vaccine stockpiles are stored at the manufacturer's facilities until their release is decided by the ICG. UNICEF procures and ships vaccines and supplies on behalf of the ICG. ICG partners on the ground (IFRC, MSF, UNICEF, WHO) support the country on vaccine logistics and roll out of immunization campaigns.
Gavi, the vaccine alliance, finances the ICG’s stockpiles of cholera, Ebola, meningitis, and yellow fever vaccines. Gavi eligible countries can receive the vaccines free of cost. Gavi eligible countries can get support for operational cost to implement the campaign.Non-Gavi-supported countries are responsible for reimbursing the cost of the vaccines and its transport, and for finding a source for the operational costs of the campaign.
Roles and responsibilities of recipient countries of ICG vaccines
The decision to release vaccine stocks is grounded in evidence-based criteria that includes epidemiological evidence of an outbreak, laboratory confirmation of pathogen, cold chain storage capacity, the country’s demonstrated capacity to conduct a vaccination campaign and an accompanying plan of action for mass vaccination. A country must submit this information in full for the request for emergency vaccine supplies to be accepted for review by the ICG members.
Once the request for vaccine supplies has been approved by the ICG members, a process is put in place to ship the vaccines and supplies. Prior to shipment, the recipient country must demonstrate that there is sufficient cold chain capacity to receive and store the vaccines and supplies. Additionally, customs and regulatory approvals must be granted and provided to UNICEF. The ICG mandate is to control quickly the outbreak and interrupt the disease transmission as soon as possible in order to save as many lives as possible. Therefore, countries are strongly encouraged to start the reactive vaccination campaign within 10 days after receiving the vaccines in the country.
ICG key performance indicators:
OpenWHO course on ICG mechanism:
Since May 2022, the online course on ICG mechanism is available. This course examines foundational understanding of the ICG and its mechanism to access the emergency vaccine stockpile for Ebola, Yellow Fever, Cholera and Meningitis. The target audience for this course is the new ICG members, Technical team members of WHO at the HQ, Regional and country office, Program managers and policy makers from Ministry of Health, National and International NGOs, donors, partners, and other UN agencies. The course is free and can be accessed using the link below:
OpenWHO course on ICG mechanism
