In collaboration with the European Commission’s Directorate-General for Health & Consumers (DG-SANCO), WHO invited institutions in various EU countries to collaborate with testing a MIM PS draft template developed by WHO, provide feedback and suggest possible modifications, as well as to collaborate on exploring methods for extracting a common learning dataset from existing patient safety reporting systems.
The project aimed to:
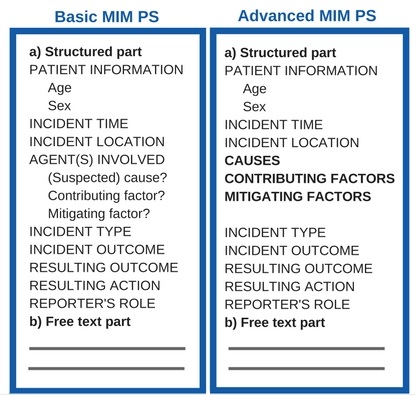
- validate the MIM PS template with user guidelines
- agree on a set of preferred terms to denominate and define the main types of patient safety incidents
- complete an EU-wide feasibility assessment by summarizing the features required by EU countries to adapt their reporting systems to, or to build new ones, based on MIM PS
- develop guidance for best practices to elicit learning from reporting systems
Results
The project was officially launched on 23 January 2014 in Brussels, in an event hosted by DG-SANCO and the Subgroup on Reporting and Learning Systems of the Patient Safety and Quality of Care Working Group.
WHO’s presentation was enhanced by the contribution of Sir Liam Donaldson, WHO Envoy for Patient Safety, who provided an overview of patient safety and reporting and learning issues.
The launch raised significant interest among the participants.