This article is part of a series of explainers on vaccine development and distribution. Learn more about vaccines – from how they work and how they’re made to ensuring safety and equitable access – in WHO’s Vaccines Explained series.
How are vaccines developed and produced?
Developing a vaccine starts with a public health need – —whether to prevent a new emerging disease or to improve current vaccines. Vaccine development happens over several phases, involving close collaboration between researchers in academia, public health institutions, regulatory agencies and pharmaceutical companies
As with all medicines, every vaccine must go through extensive and rigorous testing to ensure it is safe before it can be introduced in a country’s vaccine programme. Most vaccines have been in use for decades, with millions of people receiving them safely every yearPreclinical phase
Each vaccine under development must first undergo screenings and evaluations to determine which antigen should be used to invoke an immune response. This preclinical phase is done without testing on humans. An experimental vaccine is first tested in animals to evaluate its safety and potential to prevent disease.
If the vaccine triggers an immune response, it is then tested in human clinical trials in three phases.
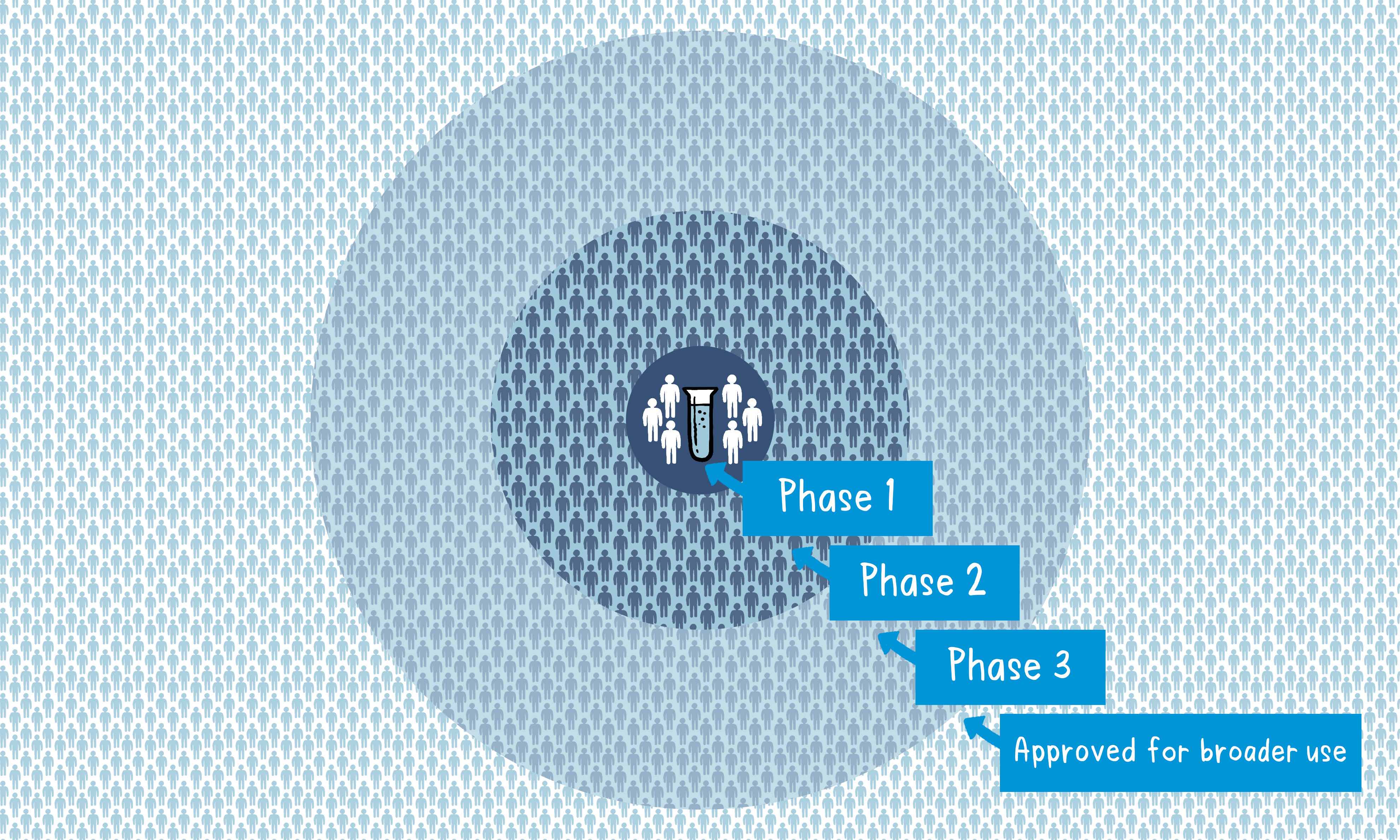
Phase 1
The vaccine is given to a small number of volunteers to assess its safety, confirm it generates an immune response, and determine the right dosage. Generally, in this phase, vaccines are tested in young, healthy adult volunteers.
Phase 2
The vaccine is then given to several hundred volunteers to further assess its safety and ability to generate an immune response. Participants in this phase have the same characteristics (such as age, sex) as the people for whom the vaccine is intended.
There are usually multiple trials in this phase to evaluate various age groups and different formulations of the vaccine. A group that did not get the vaccine is usually included as a comparator group to determine whether the changes in the vaccinated group are attributed to the vaccine, or have happened by chance.
Phase 3
The vaccine is next given to thousands of volunteers – and compared to a similar group of people who didn’t get the vaccine but received a comparator product – to determine if the vaccine is effective against the disease it is designed to protect against and to study its safety in a much larger group of people.
Most of the time, phase three trials are conducted across multiple countries and multiple sites within a country to assure the findings of the vaccine performance apply to many different populations.
During phase two and phase three trials, the volunteers and the scientists conducting the study are shielded from knowing which volunteers had received the vaccine being tested or the comparator product. This is called “blinding” and is necessary to assure that neither the volunteers nor the scientists are influenced in their assessment of safety or effectiveness by knowing who got which product.
After the trial is over and all the results are finalized, the volunteers and the trial scientists are informed who received the vaccine and who received the comparator.
When the results of all these clinical trials are available, a series of steps is required, including reviews of efficacy and safety for regulatory and public health policy approvals. Officials in each country closely review the study data and decide whether to authorize the vaccine for use.
How a vaccine is approved for production
After completing clinical trials, a vaccine must be assessed by the relevant regulatory body to ensure it meets strict standards for quality, safety and effectiveness. Once approved, manufacturers can apply for WHO prequalification (PQ), a process that evaluates the vaccine to ensure it meets global standards.
During global health emergencies such as pandemics, the WHO Emergency Use Listing Procedure (EUL) process may be used to speed up access to critical vaccines while maintaining rigorous safety checks. This fast-tracked but thorough process helps ensure vaccines reach those in need as quickly as possible, on a time-limited basis and based on a risk-versus-benefit evaluation.
The WHO PQ/EUL process helps the UN and other international procurement organizations determine whether the vaccine is suitable for use by immunization programmes.
COVID-19 vaccines: how development was accelerated
Typically, companies work independently to complete clinical development plans for a vaccine. Once a vaccine is authorized, manufacturing begins to scale up. The process, from preclinical trials to manufacturing, can take over a decade to complete.
However, in urgent situations like the COVID-19 pandemic, vaccine development can be sped up without compromising quality and safety. For COVID-19 vaccines, researchers and developers worked on several different phases in parallel, supported by unprecedented financial and political commitments. This allowed for faster development while maintaining rigorous regulatory oversight.
How vaccines are packaged
Once the vaccine has been made in bulk quantities, it is packaged in glass vials, pre-filled syringes or ampoules, and then carefully packaged for safe cold storage and transport.
Vaccine packaging must be able to withstand extreme temperatures, as well as the risks involved in being transported globally. Therefore, vaccine vials are most commonly made from glass, as it is durable and able to maintain its integrity in extreme temperatures.
How vaccines are stored
When a vaccine is too hot or too cold, it becomes less effective or even inactive. If stored at the incorrect temperature, vaccines can be ruined or unsafe for use. Most vaccines require refrigerated storage at between 2 and 8 °C. Some vaccines
require temperatures as cold as -20°C. Some of the newer vaccines need to be kept ultra cold at -80°C. For frozen vaccines some of them can be safely stored for a limited time between 2 and 8°C.
Regular refrigerators cannot maintain an even temperature consistently, so specialized medical refrigerators are required for these precious products.
How vaccines are shipped
To maintain this cold chain, vaccines are shipped using specialized equipment that does not compromise the integrity of the product. Once shipments land in the destination country, refrigerated lorries transport the vaccines from the airport to the warehouse cold room. From there, portable iceboxes are used to transport vaccines from the cold room to regional centres where they’re stored in refrigerators. If vaccination takes place outside of the regional facility, the final step often requires portable iceboxes to transport the goods to local areas for vaccination campaigns.
New technologies have invented some portable devices that can keep vaccines at their cold temperature for several days without needing electricity.
Quality control
A vaccine must be proven to be safe and effective across a broad population before it will be approved and introduced routinely into a national immunization programme. The bar for vaccine safety and efficacy is extremely high, recognizing that vaccines are given to people who are otherwise healthy and specifically free from the illness.
Further monitoring takes place in an ongoing way after the vaccine is introduced. There are systems to monitor the safety and effectiveness of all vaccines. This enables scientists to keep track of vaccine impact and safety even as they are used in a large number of people, over a long time frame. These data are used to adjust the policies for vaccine use to optimize their impact, and they also allow the vaccine to be safely tracked throughout its use.
Once a vaccine is in use, it must be continuously monitored to make sure it continues to be safe.
Once vaccines start being administered, national authorities and WHO constantly monitor for – and establish the severity of – any possible adverse side effects and responses from people who have received the vaccine. The safety of the vaccine is paramount, with regular assessments and post-approval clinical studies to report on its safety and effectiveness.
Studies are often conducted to determine how long a given vaccine remains protective.
