
Target Product Profile Directory
What is a Target Product Profile?
A Target Product Profile (TPP) indicates the characteristics required in a product to meet a particular health need. A TPP states minimal and optimal characteristics. Preferred Product Characteristics (PPC) are generally developed earlier in the development phase. A PPC describes the strategic public health goals of the product and the preferred characteristics. Target Regimen Profiles may state optimal and minimal characteristics for drug regimens.
What is the Target Product Profile Directory?
The Target Product Profile Directory is a free-to-use online database to improve the efficiency of efforts to develop new products for neglected diseases and populations as well as threats to global health. It provides a searchable database of characteristics used to describe desired health products, including medicines, vaccines, diagnostics and medical equipment. Links are provided to access the full Product Profile document. The Directory was launched in May 2019 and will be continuously updated. Users should read the FAQ and user instructions document for full background information.
The Directory does NOT aim to cover all health products. Instead it describes health product profiles for which there is no market or limited incentive for research and development. These include profiles for products to combat: the poverty-related neglected diseases, diseases identified as having the potential to cause pandemics (e.g. Ebola), anti-microbial resistance, and health issues in low- and middle-income countries.
The Directory also includes profiles for products prioritized for global action by WHO and are clearly marked as authored by WHO. The Directory also lists those product profiles authored by Product Development Partnerships, commercial companies and other organizations that share the same inclusion criteria listed above. These are labelled non-WHO profiles.
Why was the Product Profile Directory created?
The Ebola outbreak of 2014–15 and SARS-CoV-2 pandemics have highlighted the urgent need for centralized information at global level to guide and improve coordination of efforts to develop new health products for neglected diseases and populations. The initial concept for this resource is contained in the TDR report Health product research and development fund: a proposal for financing and operation (2016). Specifically, the Directory
- provides a repository for existing health product profiles;
- introduces harmonization and standardization in the description of health product profiles;
- enables a high-level landscape analysis of R&D activity related to these product profiles; and
- emphasizes access, equity and affordability as integral parts of the innovation process that need to be considered at all stages, not just after a product is developed.
What can I search for?
The Directory allows a user to search by disease, product type, date of creation, author (who produced the profile) and status (whether the profile is still active or has been archived as a historical record). The Directory provides a URL or contact email to enable the user to access, where available, the full document with more detailed information on the profile. The user can download their search results directly into a Microsoft Excel spreadsheet for further analysis.
How do I submit a new profile?
Any organization that develops a TPP that meets the inclusion criteria can submit a summary of their product profile via a form here - please contact pp-directory@who.int for more information. Please note this must include a URL linking to the publicly accessible full document of the product profile or a contact email where requests for further information can be sent. Where a collaboration has generated a product profile, a lead author of the profile must be selected.
Key Characteristics of a WHO TPP
WHO has a standardized development approach to TPPs. It involves a collaborative and consensus based approach that explicitly considers the needs and preferences of end users, such as program managers and patients, as well as subject specific experts.
All WHO TPPs are open to public comment prior to finalization and aim to illicit broad viewpoints including industry at this stage.
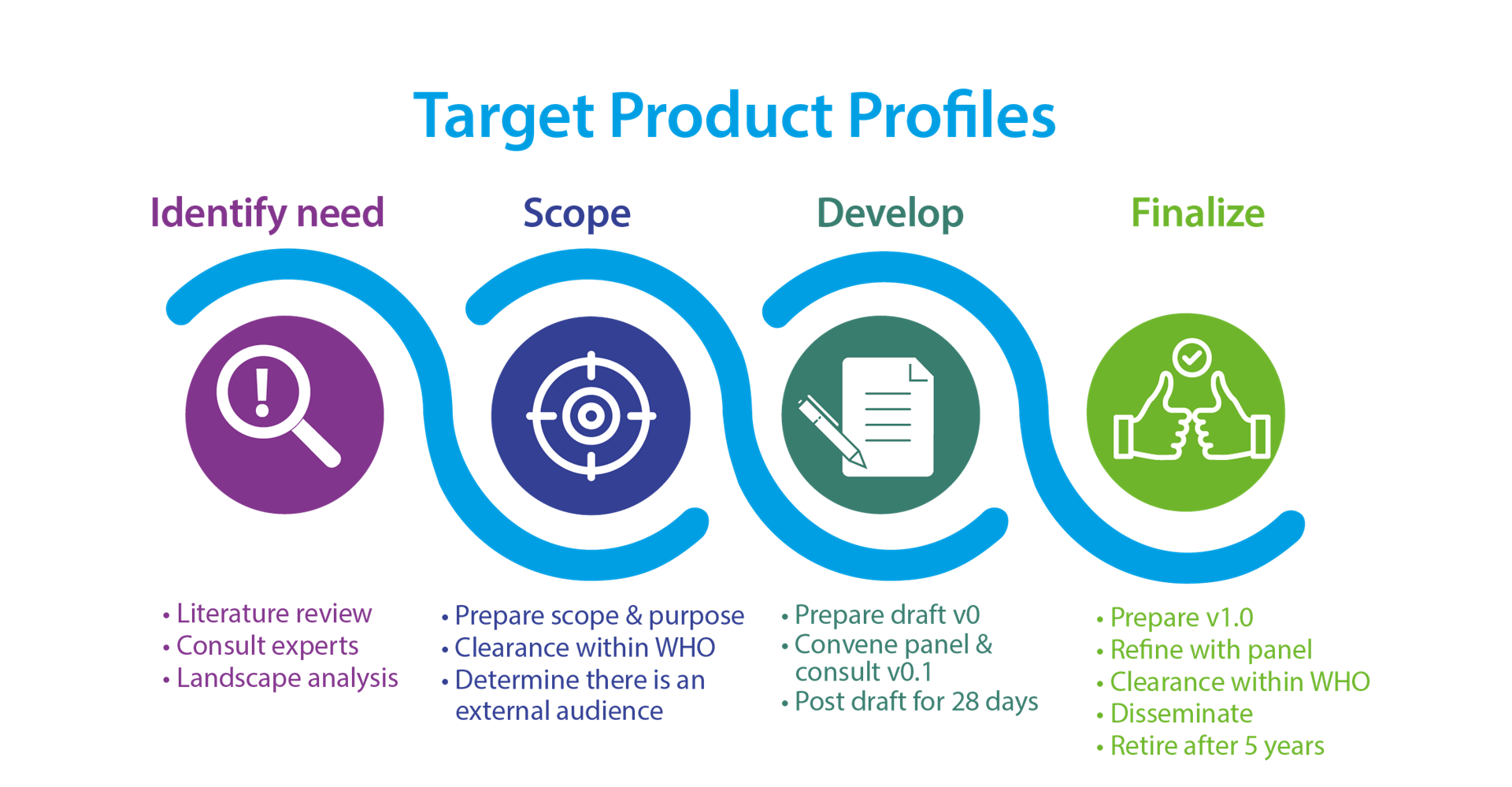
A WHO product profile
- meets a public health need;
- explicitly seeks end-user needs and preferences during development;
- is open to public comment for at least 1 month; and
- considers product developer comments separately.
To find out more about how we support Research and Development see Optimizing research and development processes for accelerated access to health products.
Selection of related publications

WHO preferred product characteristics for vaccines against Shigella
Diarrhoeal diseases are among the leading causes of morbidity and mortality worldwide. Among children younger than 5 years, it is estimated that diarrhoea...

Diagnostic target product profiles for monitoring, evaluation and surveillance of schistosomiasis control...
Health ministries currently lack effective tools for monitoring and evaluation of schistosomiasis control programmes. Egg detection can be used, but the...

This document describes World Health Organization (WHO) preferences for characteristics of monoclonal antibody (mAb) products used for passive immunization against...

BackgroundTuberculosis (TB) is a major yet preventable global health problem, with an estimated 10 million people developing TB and 1.5 million people...

The rise in antimicrobial resistance can be attributed to multiple factors: the misuse and overuse of antibiotics, the spread of infectious diseases through...

High priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting
Diagnostic manufacturers are increasingly expressing the need to be informed about the type of TB diagnostics they should invest in, as well as the potential...


