Antivenoms
For more than 100 years, the mainstay of primary treatment for snakebite has been the administration of antivenoms. Antivenoms work by boosting our immune response after a snakebite. They are made by immunizing donor animals such as horses or sheep with snake venoms. These animals have robust immune systems, and produce powerful antibodies that can bind to snake venom components, enabling our own immune defences to eliminate these toxins. Antivenoms are obtained by harvesting and then purifying the antibodies from plasma produced by the donor animal. Good-quality antivenoms can literally make a difference between life and death.
However, the potential of antivenom treatment to significantly contribute to effectively controlling the burden of snakebite morbidity, disability and mortality has been limited by a number of factors:
- Poor regulatory frameworks for antivenoms, an absence of appropriate reference standards, and a lack of expertise and capacity within national drug control laboratories;
- Inadequate investment in research and development that would lead to improved product safety, efficacy and clinical effectiveness;
- Absence of minimum specifications for neutralization of overall lethality and specific toxic activities of antivenoms that reflect clinical effectiveness definitions in defined markets;
- Traditional belief systems that associate snakebite envenoming with supernatural, rather than health-related events;
- Sustained erosion of confidence in antivenom products due to poor training of health workers, marketing of poor quality, unsafe or ineffective products, and other factors;
- Health system weaknesses, inadequate infrastructure and inefficient distribution of antivenoms;
- A cycle of consequences driven by low investment in procurement, poor quality and specificity of some antivenoms which erodes sales, drives down production, curtails profitability, driving up prices and driving down accessibility – which perpetuates market decline and drives manufacturers out of the antivenom supply sector.
The consequence of these and other issues has been most dramatic in Sub-Saharan Africa, where local manufacturing of antivenoms has always been inadequate to the needs of the continent. Major multinational antivenom producers have cited competition from inferior (and sometimes less expensive) products as the reason for their abandonment of production for Sub-Saharan Africa. The loss of effective products and their replacement with both poor quality products, and products that have not been adequately evaluated prior to market penetration, has compounded the collapse of confidence in the use of antivenoms among health workers. Furthermore, poor data on the number and type of snake bites, and difficulty in accurately establishing forward needs assessments for specific antivenom products in each country, further discourages the participation of mainstream pharmaceutical manufacturers.
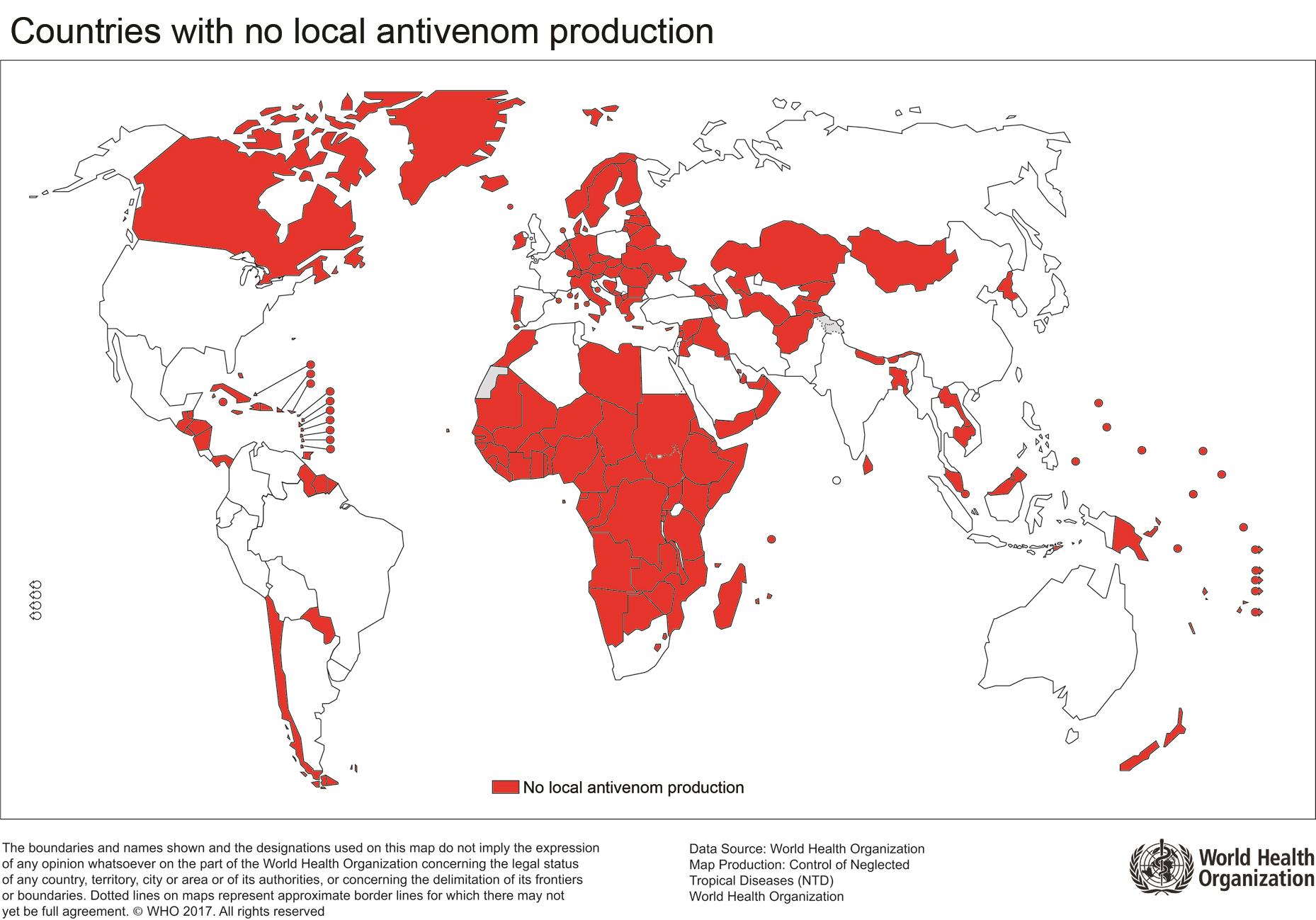
Countries with no local antivenom production
Cycle of antivenom market decline
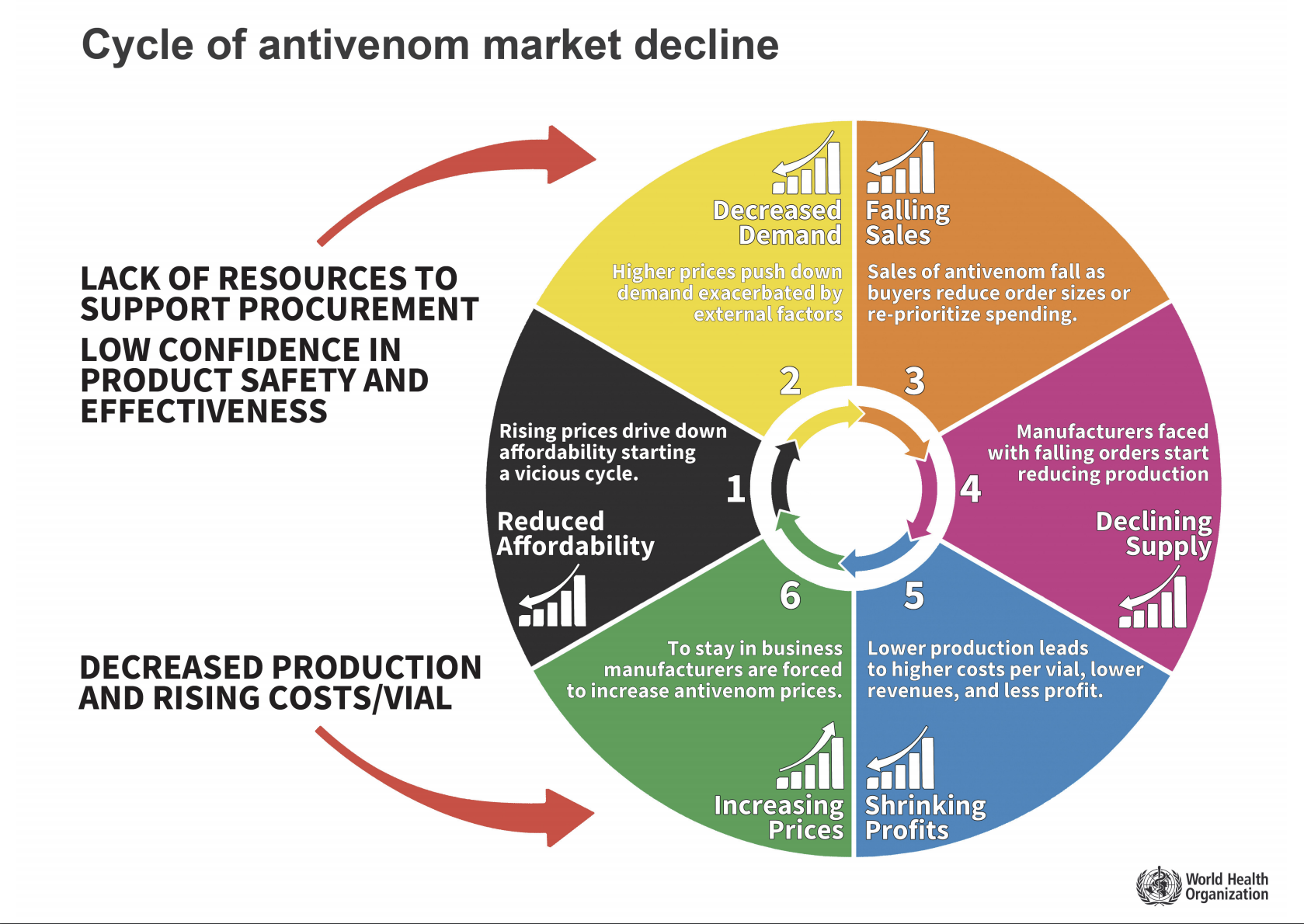
A cycle of market failure has developed in Sub-Saharan Africa making antivenom increasingly inaccessible.
Antivenoms - WHO Responses
WHO has taken important steps to respond to some of these issues:
- A structured comprehensive assessment of antivenom products intended for use in Sub-Saharan Africa commenced in 2016 and will culminate in the release of a report and specific procurement recommendations in late 2017. The key outcome of this will be an evidence-based list of WHO-recommended antivenom products that are suitable for procurement by Sub-Saharan African Member States and other stakeholders.
- Updating of the WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins in order to provide manufacturers with strong guidance on high-quality antivenom design, production, quality control, preclinical and clinical testing, and national regulatory agencies with framework guidance to ensure that products which they license meet the highest standards.
Further reform of antivenom production and regulatory governance is still required, and WHO will develop strategies to introduce programmes for further improvement in the quality and safety of antivenom products, as well as addressing some of the other constraints. Some of the approaches that may be taken include:
- Facilitation of technical support services to drive the improvement of current production and quality control technologies;
- Creation of a pathway for the entry of antivenom products into the WHO Prequalification Programme as a direct means of assuring the supply of safe, effective and affordable antivenoms to markets in Sub-Saharan Africa and Asia;
- Delivery of guidance and technical support to Member State drug regulators and Ministries of Health on the regulation and control of antivenom products.
- Establishment of a stockpile of WHO-recommended antivenoms to generate stability of supply and demand and stimulate production growth in a manner that translates into increased access to effective treatments for the victims of snakebite envenoming.
Related activity
Related health topics
