Vaccines
Vaccination is the most effective way to prevent infection and severe outcomes caused by influenza viruses.
Development and production of influenza vaccines, planning for their supplies and use as well as provision of other respective health care resources are essential components of a comprehensive seasonal and pandemic influenza response.
For more than 50 years, WHO has been collaborating with scientists and policy makers on a global scale to develop a unified approach to manufacturing, testing and regulatory oversight of influenza vaccine development as well as their efficient use and distribution.
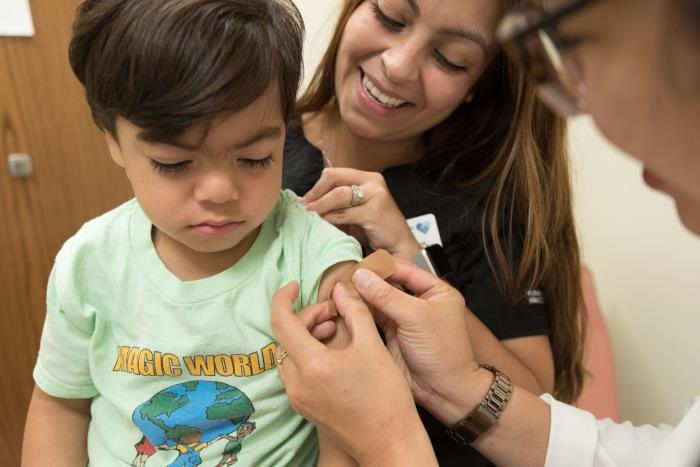
In this 2017 photo, captured inside a clinical setting, a health care provider was placing a bandage on the injection site of a child, who had just received a seasonal influenza vaccine. Children younger than 5-years-old, and especially those younger than 2-years-old, are at high risk of developing serious flu-related complications. A flu vaccine offers the best defense against flu, and its potentially serious consequences, and can also reduce the spread of flu to others.
Influenza vaccine viruses and reagents
The constantly evolving nature of influenza viruses requires continuous global monitoring and frequent reformulation of influenza vaccines.
The World Health Organization (WHO) convenes technical consultations in February and September each year to recommend viruses for inclusion in seasonal influenza vaccines for the northern and southern hemispheres, respectively. These recommendations are based on information provided by the WHO Global Influenza Surveillance Network (GISN), now the WHO Global Influenza Surveillance and Response System. Since 2004, influenza A(H5N1), A(H9N2) and other subtypes of influenza viruses have also been taken into consideration by GISRS for pandemic preparedness purposes.
The development of high yield candidate vaccine viruses is a complex process, involving collaboration of laboratories involved in developing reassortants and WHO Collaborating Centres (CCs). Two technologies are currently being used: classical reassortment (available since 1971) and reverse genetics, a patent technology.
Once developed, these candidate reassortants are sent to WHO CCs for characterization of their antigenic and genetic properties before being released to interested institutions on request. Reference reagents are subsequently developed and standardized by Essential Regulatory Laboratories (ERLs), in collaboration with vaccine manufacturers and made available to manufacturers worldwide upon request.
Processes
Why influenza vaccine viruses need to be updated?
- Influenza vaccination is currently the principal means of reducing or counteracting influenza mortality and morbidity burden in the community.
- The constantly evolving nature of influenza viruses requires continuous global monitoring and frequent reformulation of influenza vaccines.
- Rapid spread of influenza viruses during seasonal epidemics and occasional pandemics tightly frames the whole process if vaccine is to be manufactured and delivered on time.
- A prerequisite of production and supply of an optimal influenza vaccine is the selection and development of optimal candidate vaccine viruses, and the development and availability of vaccine potency reagents.
Selection of vaccine viruses
- Vaccine virus selection has been conducted by the WHO Global Influenza Surveillance and Response System (GISRS) since 1973.
- National Influenza Centres (NICs) of GISRS conduct virological surveillance at national level with more than 600,000 clinical specimens tested annually since 2007.
- Representative specimens and virus isolates are sent to WHO Collaborating Centres (CCs) with more than 5,000 virus isolates characterized by CCs yearly.
- Collaborative serological studies have been conducted by WHO CCs and Essential Regulatory Laboratories (ERLs) using sera from vaccinees, to determine whether the antibody levels produced by current vaccines are able to react sufficiently against circulating influenza viruses.
- Twice yearly WHO organizes consultations with experts from WHO CCs, ERLs and other partners to review data generated by GISRS and makes recommendations on influenza vaccine composition for the next northern or southern hemisphere influenza seasons.
Development of candidate vaccine viruses
- In general influenza wild type A viruses recommended for inclusion in vaccines do not grow efficiently in eggs for large scale production.
- Two technologies are being used for the development of suitable candidate reassortant vaccine viruses:
- classical reassortment, available since 1971 to generate hybrid viruses.
- reverse genetics, a patented technology, available to attenuate highly pathogenic viruses and reassort the attenuated HA and NA with backbone virus.
Vaccine potency reagents
- Reference antigen and sheep antisera are developed by ERLs in collaboration with vaccine manufacturers, standardized by ERLs, and made available to manufacturers worldwide on request.
