Medical devices for patient management
This page includes the latest guidance on medical devices for the COVID-19 response (including all relevant technical specifications), resources for oxygen therapy and a health facility readiness tool. The page is structured as follows:
- Priority medical devices list for the COVID-19 response and associated technical specifications
- Resources for Medical Oxygen
- Facility readiness inventory tool
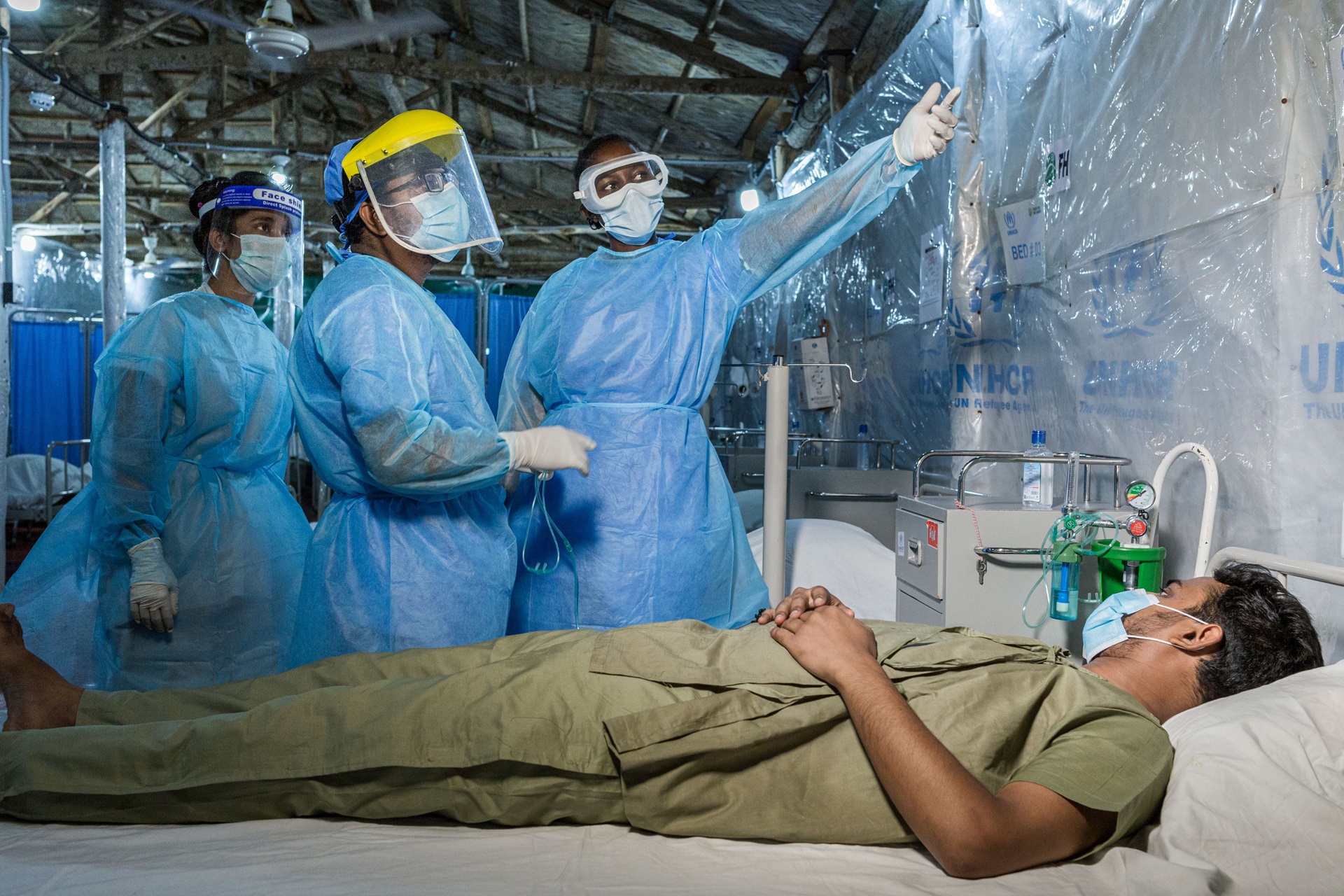
COVID-19: BANGLADESH. WHO is supporting COVID-19 preparedness and response for vulnerable Rohingya refugees and host communities in Cox’s Bazar, Bangladesh.

Priority medical devices list for the COVID-19 response and associated technical specifications
This document describes the medical devices required for the clinical management of COVID-19, selected and prioritized according to the...
Resources for Medical Oxygen
Oxygen sources and distribution for COVID-19 treatment centres
This interim guidance on medical oxygen sources and distribution strategies for COVID-19 treatment has been adapted from WHO and UNICEF’s technical specifications and guidance for oxygen therapy devices, which is part of the WHO medical device technical series. It describes how to quantify oxygen demand, identify oxygen sources that are available, and select appropriate surge sources to best respond to COVID-19 patients’ needs, especially in low-and-middle income countries.

Other relevant technical specifications


WHO technical specifications for oxygen concentrators
Facility readiness inventory tool
Countries can use this tool to collect in-depth facility inventories of biomedical equipment re-allocation, procurement and planning for COVID-19 case management. The survey assesses quantified availability and the causes for non-functioning of different sources of oxygen delivery and supply systems to the patient in order to determine priorities and re-allocation requirements in accordance with needs. Content areas include:
- Oxygen supplies and equipment
- Respiratory instruments and equipment
- Suction devices
- Ventilators
- Autoclaves/sterilizers

Related Health Topics

