Tools and Innovations in Pharmacovigilance
Pharmacovigilance is the science and activities related to the detection, assessment, understanding and prevention of adverse effects or any other possible medicinal product-related problems.
A pharmacovigilance system is a system used by a country and its authorized technical entity to fulfil its legal obligations and responsibilities in relation to pharmacovigilance. It is designed to monitor the safety of authorized medicinal products and detect any change to their risk-benefit balance. Such a system is an essential component of healthcare and rational use of medicinal products. It is also diversely referred to as adverse event monitoring, safety surveillance, spontaneous reporting, post-marketing surveillance or variations of these.
Such systems are needed because the information obtained during clinical trials of new medicinal products is by design insufficient to provide a comprehensive overview of its safety and effectiveness in routine clinical practice (limitations of pre-licensure clinical studies that have certain limitations for example short duration, small numbers of individuals, exclusion of certain individuals with other diseases, exclusion of pregnant women, infants and the elderly). The long-term safety of a medicinal product is thus only known when the medicinal product is being used widely in a population and its safety is being monitored by organized local, national and international efforts.

The pharmacovigilance cycle includes identification of adverse events, followed by its notification, reporting of the event to the health system using standard reporting tools and mechanisms, followed by investigation on the nature of the event, determining its cause and providing feedback to all stakeholders in the cycle. The data collected during this process is processed and carefully analyzed to look for unusual patterns. Communication also plays a key role in the entire pharmacovigilance process.
Each element in the pharmacovigilance cycle is important. WHO has developed tools and techniques for each of them and introduced innovations for their use. To refine and improve tools and innovations in pharmacovigilance, several strategies and approaches have been adopted. Some of these have been developed already, some are currently under development, and some are being conceptualized.
Tools and innovations used currently
Identifying and standardizing the core data elements for safety
WHO in discussion with the member states and guided by the WHO Global Advisory Committee on Vaccine Safety (GACVS) identified the 25 core variables for reporting of Adverse events following immunization (AEFI). These core data elements are critical to ensure global standardization of reporting and analyses and have been included in the reporting forms of several countries. Using the core variables, the WHO Standard AEFI reporting form has been developed. A similar mechanism for reporting adverse drug events is also being currently developed.
Digital solutions for reporting adverse events customized to local needs
Building on the Vigflow, the electronic data collection system available to all countries in WHO PIDM, to facilitate reporting of adverse events following immunization (using the 25 core variables) by healthcare professionals, patients, and other stakeholders, WHO has developed user-friendly mobile applications and web platforms. The VigiFlow for AEFI is such a system for collecting and analyzing reports of possible side effects from vaccines and is a vaccine surveillance platform built for AEFI data management and analysis.
Using VigiFlow for AEFI integrated with VigiMobile (a mobile phone app specifically developed for national immunization programmes), immunization field workers can quickly and accurately report AEFI on their smartphones or other mobile devices regardless of internet access. Thus, a complete system for managing adverse events following immunization (AEFI) is able to seamlessly collect and transmit AEFI data from the end user to the supervisory levels at the district, province and national levels and share the same to the WHO global database for the WHO Program for International Drug Monitoring (PIDM).
To facilitate suspected ADR reporting in countries, WHO is currently developing the core variables and a standard reporting form for reporting ADR. The reporting of ADR will be supported by use of similar electronic tools via Vigiflow for ADR and Vigimobile for ADR.
Data Integration and Standardization
Implementation of standardized data collection methods and terminology across different healthcare systems to enable more effective data aggregation and analysis. As case-based data is confidential and should be encrypted when shared with WHO, E2B(R3) encoding is needed for electronic transmission of Individual Case Safety Reports (ICSR). WHO has been working with several countries and several database solutions to assist with interoperability of the in-country software solutions to share data with the WHO global database (VigiBase).
Real-time Monitoring
WHO has empowered countries to establish real-time monitoring systems that can quickly identify and assess emerging safety concerns. Using VigiLyze, that uses advanced analytics, key national focal persons of the National Regulatory Authority (NRA) and the Expanded Program on Immunization (EPI) in WHO PIDM members continuously analyze data from various sources and monitor adverse events to identify trends that may indicate emerging safety issues in medicines and vaccines.
Investigation and causality assessment of adverse events - Innovative decision-making electronic tools
To facilitate complex processes such as AEFI investigation and AEFI causality assessment, WHO has developed an electronic tool for investigation of serious AEFI cases. The AEFI investigation software provides an investigator with tools such as the AEFI reporting form, the AEFI investigation form and guidance on investigation using vaccine reaction rates information sheets, aide memoir on AEFI investigation the Brighton collaboration case definitions, the WHO vaccine position papers and finally assists the sharing of the investigation dossier to the national managers for causality assessment.
The AEFI causality assessment software offers a systematic documented method for standardized global causality assessment for individual serious adverse events following immunization (AEFI). It is intended to be used by staff at national level (such as members of national AEFI committees) and at subnational level, as well as immunization programme managers and field staff. By integrating the AEFI causality assessment software with eLearning[10], it also serves as an educational tool for trainers and researchers and as a ready reference guide on all facets of AEFI – reporting, investigation, data management and causality assessment.
Advanced Analytics and Visualization
Annually, WHO develops Global and Regional maps and charts for vaccine safety that provide insights into monitoring AEFI reports and trends based on performance indicators developed by WHO. Such visualization of vaccine safety data provides perceptions in an actionable format for healthcare professionals, regulators, and other stakeholders.
Education and Training
A plethora of innovative training courses on medicines and vaccines safety with multiple learning approaches such as didactic approach, game based eLearning approach and hybrid courses are offered by WHO to provide training to healthcare professionals and patients about pharmacovigilance principles, reporting procedures, and the importance of timely reporting. WHO has also developed slide decks and training materials on vaccine and medicines safety that are available to program managers and regulators on request. They can be customized to the local context and used. For more information please visit our "Training resources" page.
Patient Engagement
People using health services are increasingly asking for more responsive, open and transparent health care systems. They expect practitioners to engage them in the decision-making process. Engaged patients are better able to make informed decisions about their care options. The innovative tools developed by WHO can be customized for setting up national or local reporting systems where safety data can be appended using tools to report adverse events.
Global Collaboration
Guided by member states and its global committees (GACVS and ACSoMP), WHO has developed tools and innovations that foster collaboration between national and international regulatory agencies, pharmaceutical companies, healthcare providers, and patient advocacy groups to share information and expertise. This enables pharmacovigilance systems to become more proactive, efficient, and responsive, leading to improved patient safety and better management of vaccines and medication-related risks.
Tools and innovations in the pipeline
Artificial Intelligence (AI) and Machine Learning
Efforts are ongoing to develop AI models that can predict potential adverse events based on patient profiles, medical history, and genetic factors and utilize machine learning to identify patterns and associations between medications and adverse events that might not be immediately obvious.
Collaborative Platforms
WHO has been working with internal teams and the GACVS to create platforms and database dashboards that will allow healthcare professionals, researchers, and regulators to collaborate and share safety data and insights in real-time. Encouraging international collaboration by sharing anonymized data across borders to identify global safety trends is critical to ensure that relevant stakeholders are prepared for addressing safety signals and also self-evaluate the performance of their own national pharmacovigilance systems.
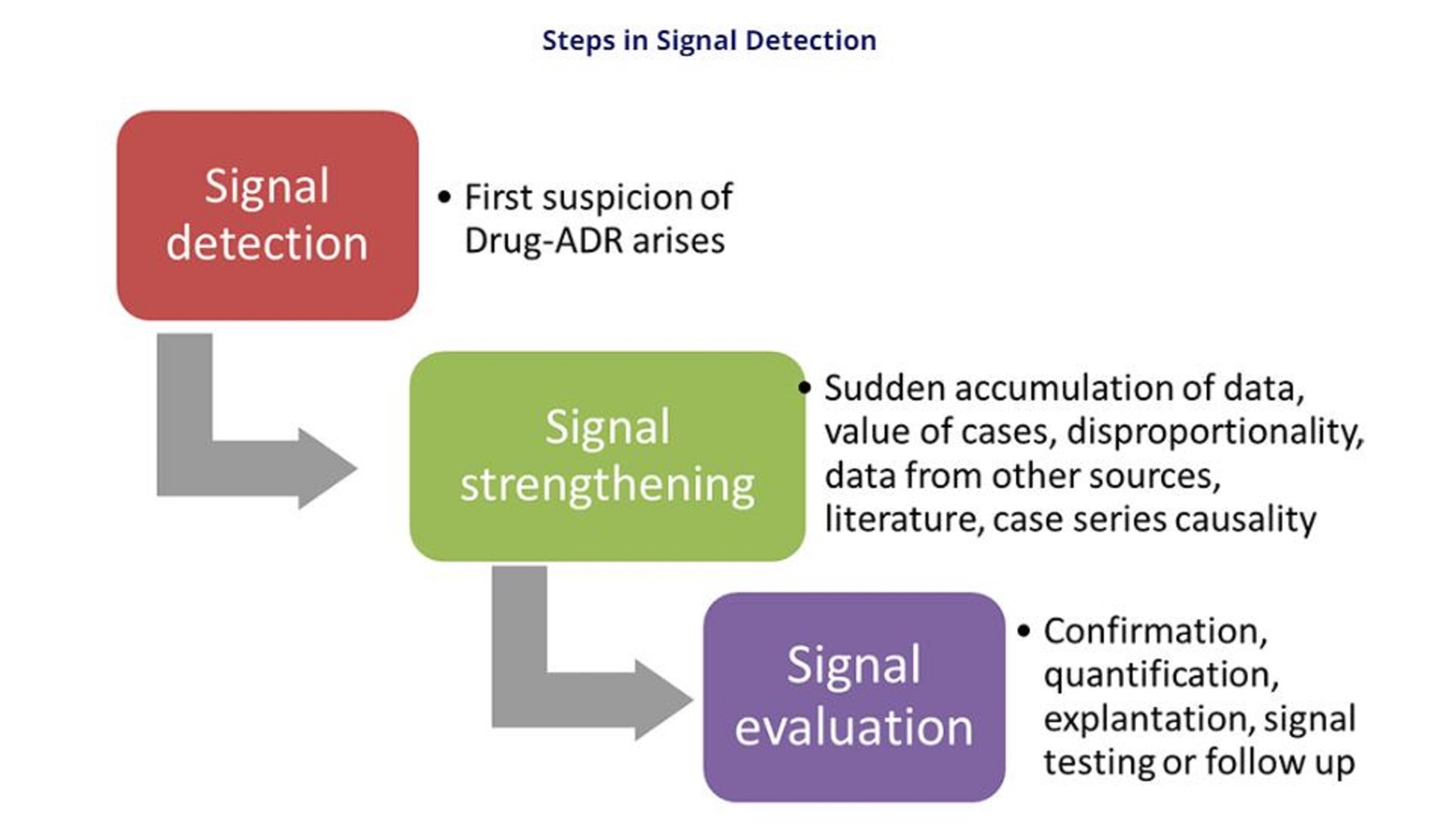
Advanced Signal Detection
WHO Program for International Drug Monitoring (WHO PIDM) is coordinated by the WHO collaborating centre in Uppsala, Sweden (Uppsala Monitoring Centre or UMC). UMC has developed advanced statistical methods and algorithms to detect signals of potential adverse events more accurately and efficiently. VigiBase, with over 36 million ICSRs has an ocean of information. By incorporating Bayesian modeling and data mining techniques, signal detection capabilities have been enhanced.
Natural Language Processing (NLP)
Using the free text field in VigiFlow, efforts are ongoing to implement NLP algorithms to extract relevant information from unstructured text in electronic health records, medical notes, and ICSRs, enabling more efficient adverse event identification.
