Gonococcal antimicrobial resistance in the Western Pacific Region

Neisseria gonorrhoeae is a human pathogen that causes gonorrhoea. Most commonly transmitted through sexual contact, the infection also can be passed from a mother to her baby during childbirth causing gonococcal eye disease. The World Health Organization estimates that there were 87 million new N. gonorrhoeae infections globally in persons aged 15–49 years in 2016, with 27% of the global burden of this disease estimated to be in the Western Pacific Region (1). As there is currently no vaccine to prevent gonococcal infection, disease control strategies are based on effective treatment of patients and their partners with antibiotics. Antimicrobial resistance (AMR) is a longstanding and serious problem in N. gonorrhoeae. The emergence of resistance to all classes of antibiotics used in first-line therapy has been reported globally (2) since antibiotics were first introduced for the treatment of gonorrhoeae in the 1930s (3). Antibiotic classes that have been in first-line clinical use for gonorrhoeae and for which resistance has emerged include the sulphonamides, penicillins, tetracyclines, macrolides, fluoroquinolones and early-generation cephalosporins. The combination of widespread AMR and the high variability of circulating strains of N. gonorrhoeae continues to compromise gonococcal disease control (2).
In the Western Pacific Region and elsewhere, ciprofloxacin and penicillin resistance in N. gonorrhoeae is high and widespread. The current first-line treatment in most countries and areas is ceftriaxone or cefixime, plus azithromycin, which is in line with the 2016 WHO guidelines for the treatment of gonorrhoea (https://www.who.int/publications/i/item/9789241549691).
Isolates with decreased susceptibility to ceftriaxone and azithromycin have been widely reported (http://iris.wpro.who.int/handle/10665.1/14324).

IM, intramuscular injection; PO, oral administration; g, gram; mg, milligram;
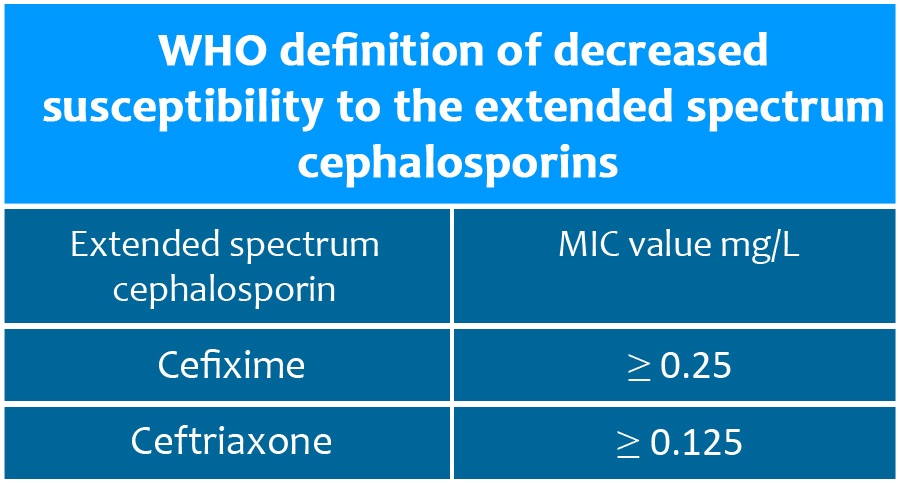
MIC, minimum inhibitory concentration; mg/L, milligram per litre
Source: WHO global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. Geneva: World Health Organization; 2012. In early 2018, one infection with a gonorrhoea strain resistant to ceftriaxone (MIC value 0.5 mg/L) and with high-level resistance to azithromycin (MIC value > 256 mg/L), as well as to most other antibiotics, was reported in the United Kingdom of Great Britain and Northern Ireland (4). Two such strains were also reported in Australia (4). Two of the three cases were travel-associated, and both acquired gonorrhoea in the South-East Asia Region. Antimicrobial susceptibility data are lacking in many countries and areas in this Region.
Fact sheet
__________________________________________
Gonococcal antimicrobial resistance factsheet (2021)
Gonococcal antimicrobial resistance factsheet (2017)
Table 1. Gonococcal AMR in selected countries and areas in the Western Pacific Region, 2015-2016
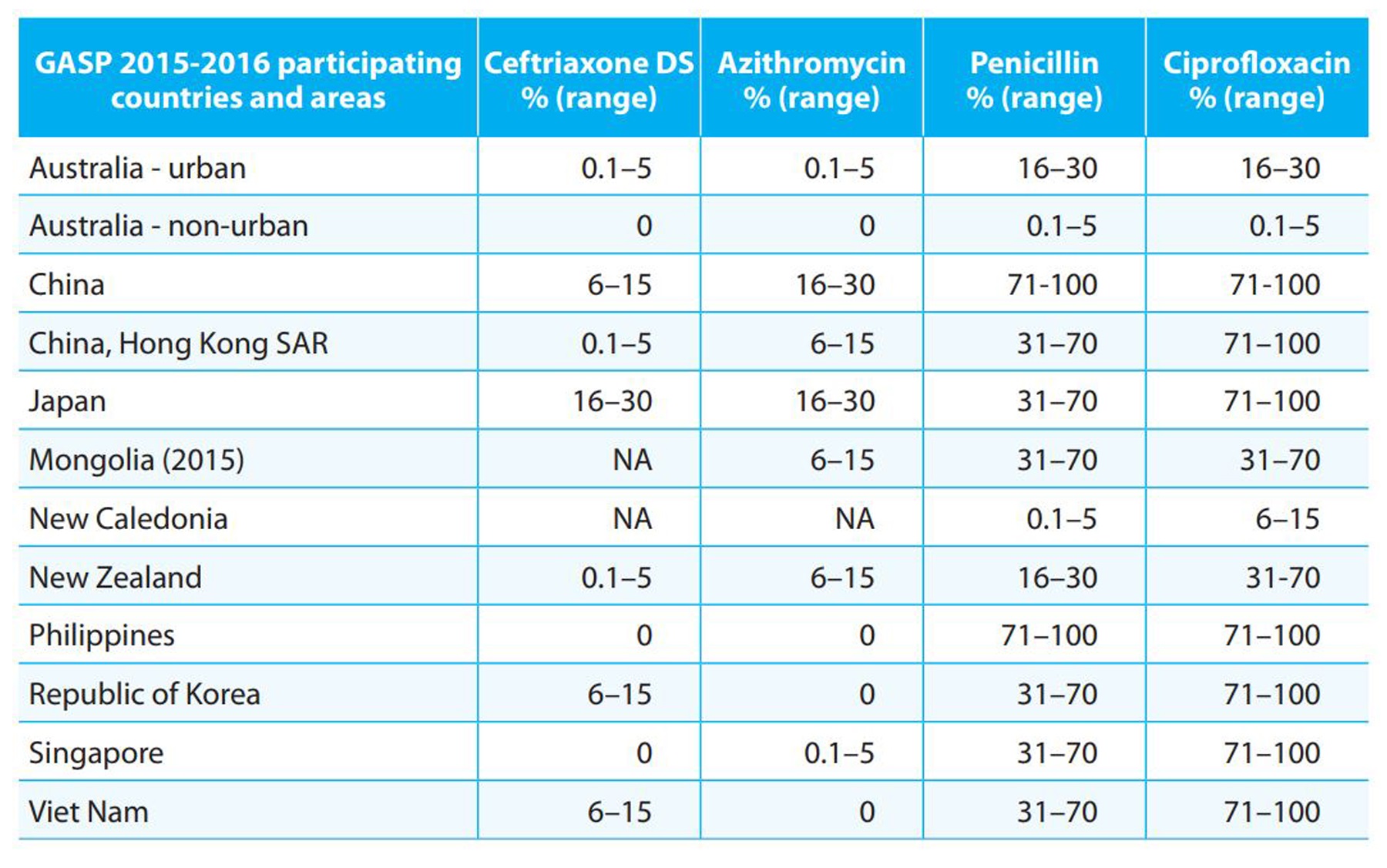
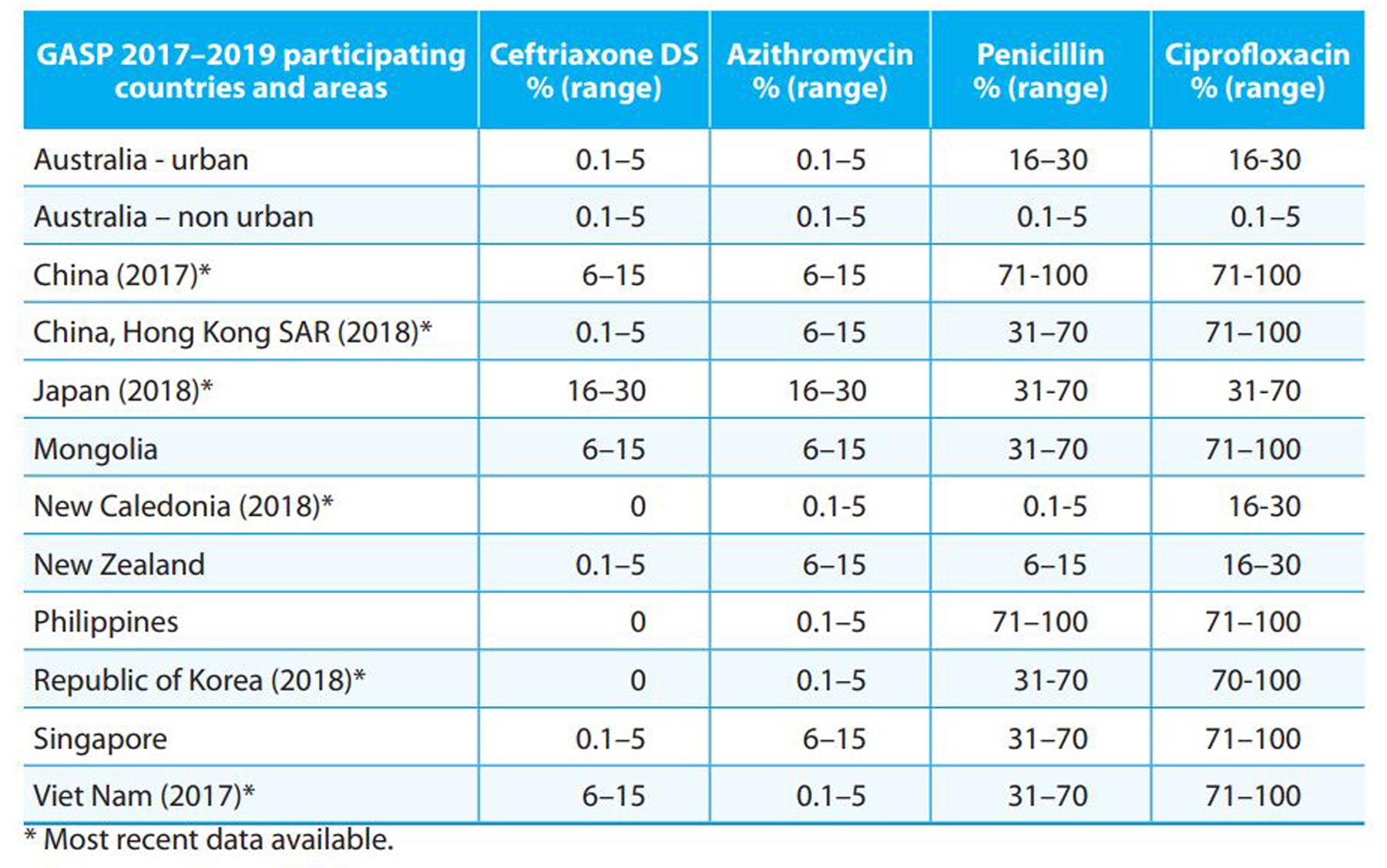
GASP, WHO Gonococcal Antimicrobial Surveillance Programme; DS, decreased susceptibility (MIC value ≥ 0..125 mg/L).
Data are for 2016 unless otherwise stated.
Table 2. Gonococcal AMR in selected countries and areas in the Western Pacific Region, 2017-2019
The Gonococcal Antimicrobial Surveillance Programme
The WHO Gonococcal Antimicrobial Surveillance Programme (GASP) was established in 1992 and is a worldwide laboratory network that is coordinated by regional coordinating centres across the WHO regions. The programmes objectives are:
- to ensure adequate sentinel gonococcal AMR surveillance in order to inform treatment guidelines in all countries.
- to establish a strategy to rapidly detect patients with gonococcal infections who experience clinical and/or microbiological treatment failure following treatment with recommended cephalosporin therapy; and
- to ensure the effective clinical management of infected patients and their sexual partners.
The GASP regional focal points and participating countries work together to enable the programme activities. From the Western Pacific Region, Australia, Brunei Darussalam, Cambodia, China, Fiji, Hong Kong SAR (China), Japan, Malaysia, Mongolia, New Caledonia, New Zealand, Papua New Guinea, the Philippines, the Republic of Korea, Singapore, Tonga and Viet Nam have participated in GASP.
For more on GASP, see
https://www.who.int/initiatives/gonococcal-antimicrobial-surveillance-programme
_resistance/en/
At the country level, the aims of GASP include collecting at least 100 representative gonococcal isolates per year, ideally tested for AMR susceptibility using quantitative methods for determination of MIC and using validated and standardized interpretative criteria, to finally inform treatment guidelines (see https://www.who.int/data/gho/data/themes/topics/who-gonococcal-amr-surveillance-programme-who-gasp).
The regional coordinating laboratories provide technical advice and support and conduct quality assurance activities to support GASP.
Enhanced Gonococcal Antimicrobial Surveillance Programme
The Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP) is a collaboration between the World Health Organization and the United States Centers for Disease Control and Prevention (CDC) established in 2016. EGASP aims to monitor trends in antimicrobial drug susceptibilities in N. gonorrhoeae by using standardized sampling and laboratory protocol to improve quality, comparability and timeliness of gonococcal antimicrobial drug resistance data across multiple countries. It also aims to inform country-specific treatment guidelines by assessing resistance patterns in key populations at highest risk for antimicrobial drug-resistant gonorrhoea. The first EGASP site was established in Thailand in 2015 and the first in the Western Pacific Region was established in the Philippines in 2017, with ongoing development in other Member States.
For more on EGASP, see https://wwwnc.cdc.gov/eid/article/23/13/17-0443_article
Global Antimicrobial Resistance Surveillance System
The Global Antimicrobial Resistance Surveillance System (GLASS) was launched in October 2015 to support the WHO global action plan on AMR. Its aim is to support global surveillance and research to strengthen the evidence base on AMR, inform decision-making and drive national, regional and global actions.
GLASS initially focused on surveillance data on human priority bacterial pathogens considered to present the greatest threat globally, which include N. gonorrhoeae. There is thus overlap between GLASS and GASP, with both systems monitoring AMR in N. gonorrhoeae. Ultimately, GASP is a part of GLASS and their consolidated data are incorporated in the WHO Global Health Observatory (see https://www.who.int/data/gho/data/themes/topics/who-gonococcal-amr-surveillance-programme-who-gasp).
GLASS is evolving and progressively incorporating data from other surveillance systems related to AMR in humans, including antimicrobial usage, foodborne AMR and surveillance of health care–associated infections.
For more on GLASS, see https://www.who.int/initiatives/glass.




