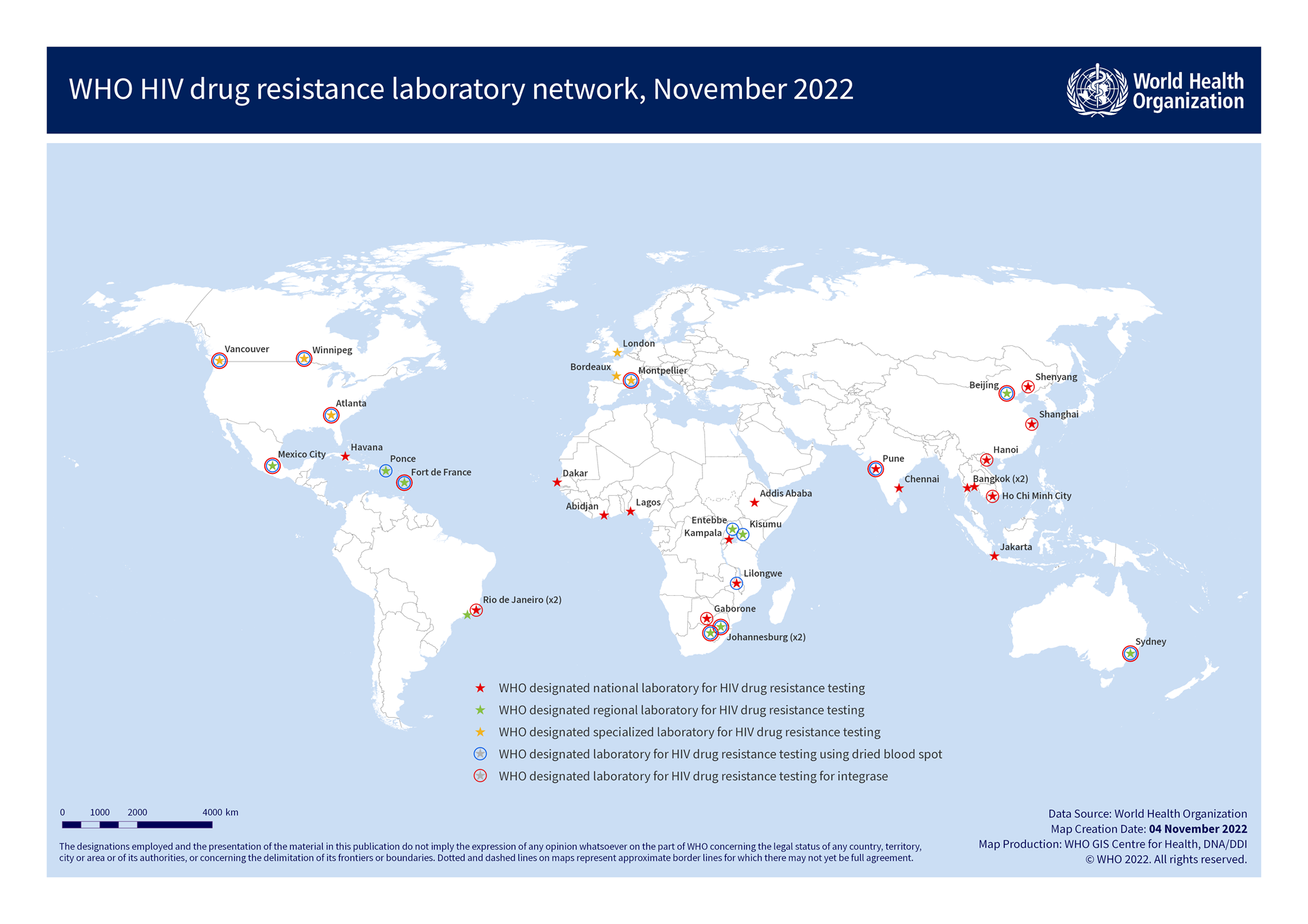
Laboratory network
The WHO HIV drug resistance laboratory network supports HIV drug resistance surveys by providing accurate and timely genotyping results that meet WHO specifications.
Its objectives are to ensure:
- the proper collection, handling, shipment and storage of specimens; and
- the availability of quality-assured HIV genotyping laboratory services producing comparable and reliable results at the national, regional and global level.
The national HIV drug resistance working groups that coordinate WHO-recommended surveys must use a WHO-designated genotyping laboratory to provide quality-assured testing services for drug resistance surveys.
To be designated as a laboratory for WHO surveys, several mandatory criteria must be met including a site inspection and annual external proficiency testing. The roles and responsibilities of network laboratories, recommended testing, internal and external quality assurance procedures, and assay validation recommendations are summarized in the WHO/HIVResNet HIV drug resistance laboratory operational framework.

Minimum criteria for external quality assurance proficiency tests for use by laboratories...

Target product profile for HIV drug resistance tests in low- and middle-income countries:...

The WHO HIV Drug Resistance Network (HIVResNet) HIV drug resistance laboratory operational framework describes how WHO HIVResNet laboratories function...
List of WHO-designated HIV Drug Resistance laboratories (pdf, 178 KB)
Laboratories seeking designation as a WHO HIV drug resistance laboratory must be nominated by the health ministry of the country in which they are located. They are encouraged to complete the application form and provide the required supporting documentation.
WHO HIVDR Genotyping Laboratory Questionnaire (word document, 54 KB)
HIV drug resistance testing using dried blood spot
A set of protocols for collecting and analysing dried blood spots for drug resistance testing was developed by the HIVResNet dried blood spots working group. These protocols are based on standard procedures used in laboratories that performed well during an assay validation exercise carried out in the WHO HIVResNet Laboratory Network.