
Research
A lady lab technician is performing research and technology work in karachi, Pakistan.
Research, development and innovation are fundamental enablers of programmatic progress for all NTDs. Adequately structured operational and implementation investigations, including community-based and applied research, are essential for building a solid foundation on which effective interventions can be designed and delivered.
Innovations in diagnostics
A priority area
Diagnostics constitute a priority area in terms of advancing the global NTD agenda, as they directly inform many of the targets set out in the 2021–2030 road map, Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021−2030
As the intensity of infection and prevalence of NTDs progressively decrease, current methods of diagnosis may not have the necessary sensitivity or specificity to support programmes through to the point of target delivery. New testing techniques and the possibility of integrating diagnostics for multiple NTDs into single platforms will need to be investigated. New tests that will be required to support decisions that include:
- changing the frequency of mass drug administration;
- deciding to stop mass drug administration and commence surveillance; and,
- validating or verifying elimination and/or eradication of a disease.
In many cases, diagnostics may require accurate point-of-care tests that can be deployed in resource-limited settings in or near remote communities to overcome key challenges such as delayed healthcare-seeking behaviour, lack of disease awareness and population movement.
Diagnostics Technical Advisory Group (DTAG)
WHO has established a Diagnostics Technical Advisory Group (DTAG) for Neglected Tropical Diseases to address priority areas for NTD diagnostics, to identify gaps in access to currently available tools, and to advise on the innovations and developments required to ensure that the diagnostic process is able to properly inform decisions about work to combat NTDs.
The DTAG has also instituted several focus-specific subgroups to report on the key issues faced by the NTD community in areas such as monitoring and evaluation, manufacturing and regulatory pathways, and clinical diagnosis, imaging, and microscopy.
Substantial progress has been made since 2019 and this has boosted the confidence of partners and stakeholders in supporting the development of new diagnostic tools.
Target product profiles for diagnostics
Supported by the DTAG and its subgroups, WHO is leading the way in developing target product profiles (TPPs) for diagnostics.
TPPs outline the desired characteristics of a product that is aimed at a particular disease or diseases. TPPs state intended use, target populations and other desired attributes, including safety and efficacy-related characteristics. Such profiles can guide product research and development (R&D). TPPs help define the type of diagnostics that would be most supportive of the aims and targets envisaged by the road map for neglected tropical diseases 2021–2030.
TPP Dashboard
The TPP dashboard provides an at-a-glance view of the status of Target Product Profiles for the portfolio of NTDs, showing those already and those currently either in development or awaiting public consultation.
Target product profile for vector control
The Aedes aegypti mosquito is the principal vector of dengue, chikungunya, Zika and yellow fever viruses. Dengue incidence has been rising significantly since 2000.
Effective control of this vector is difficult to achieve and sustain, given the mosquito’s high reproductive rate and adaptation to urban habitats. The mosquito eggs can survive desiccation and the larvae develop in artificial containers, including cryptic or hard to find habitats in the urban environments.
A novel approach of sustainable control of this species was recently demonstrated using bacteria belonging to the genus Wolbachia. Aedes aegypti mosquitoes infected with Wolbachia spp. are less susceptible to dengue virus infection.
The TPP was recently developed for Wolbachia product(s) that would trigger a replacement in the population of Aedes aegypti, thus enabling countries to achieve reductions in the dengue burden at national and global level.
The TPP defines the performance and cost characteristics of Wolbachia and is meant to guide product developers, national regulators, and their potential funders to assess candidate products to determine whether they meet, or have the potential to meet, the requirements.
Target product profile for snakebite antivenoms
Countries in the WHO Region for Africa are heavily impacted by snakebite envenoming.
WHO is prioritising prevention and control activities in this region to improve access to safe, effective and affordable antivenoms.
WHO is currently involved in the following:
- development of the first public antivenom Target Product Profiles (TPP) for sub-Saharan Africa
- assessment of the establishment of an antivenom stockpile programme in West Africa, and
- development of a community engagement toolkit that can be piloted in selected countries of the Region.
Latest TTPs related to NTD
All →
Diagnostic target product profiles for trachoma surveillance

In 2007, the Sixtieth World Health Assembly adopted resolution WHA60.13 on control of leishmaniasis, urging Member States, among other actions: to strengthen...

Leishmaniasis is caused by protozoan parasites which are transmitted by the bite of infected female phlebotomine sandflies. The disease is poverty-related...
Innovations in medicines
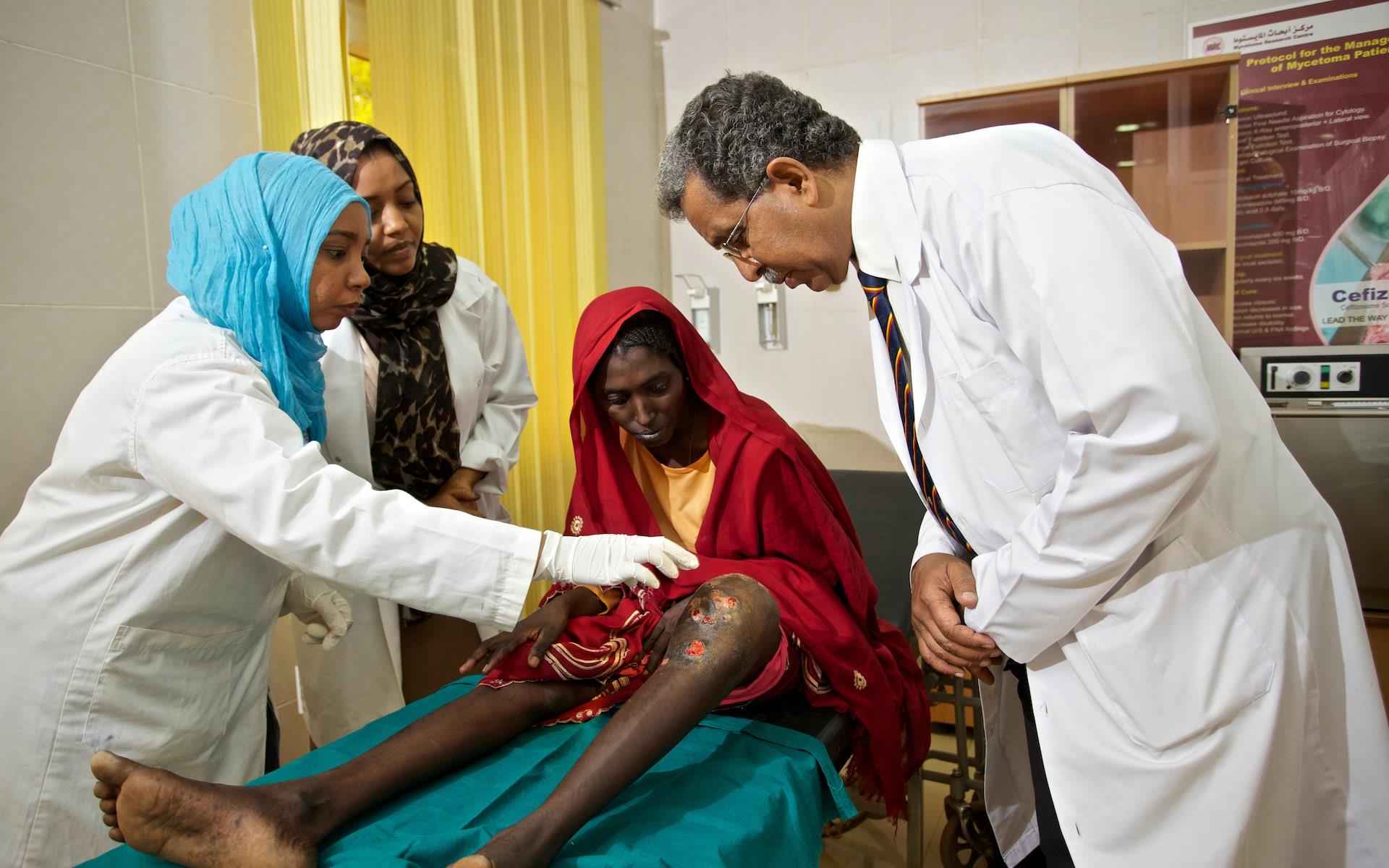
A Mycetoma patient, Almina Yusuf Hassan Balel with an infected leg in Sudan
Fosravuconazole for mycetoma
Recent innovations in the treatment of fungal mycetoma are distinctly encouraging. Currently available medicines for eumycetoma, itraconazole and ketoconazole, are expensive and not universally effective. Use of ketoconazole is also limited by its severe liver toxicity.
Fosravuconazole was developed by Eisai Ltd (Japan) and could prove to be an effective and affordable treatment for eumycetoma – potentially engineering a completely new perspective for patients suffering with this debilitating disease.
Fexinidazole for HAT
Human African trypanosomiasis, also known as sleeping sickness, is another field in which innovations could lead to significant improvements in available treatments. WHO has been working closely with the Drugs for Neglected Diseases Initiative to develop new treatments for both stage 1 (early stage, haemolymphatic) and stage 2 (late stage, meningoencephalitic) of the disease.
In 2019, fexinidazole was added to WHO’s List of Essential Medicines for adults and children. Clinical trials are currently underway in Malawi and Uganda to evaluate treatment for the Rhodesiense form of the disease.

Paediatric Praziquantel for schistosomiasis
WHO is working closely with several organizations and partners to reduce the burden of schistosomiasis in children living in endemic countries globally.
Praziquantel is a WHO-recommended medicine for the treatment of schistosomiasis for all ages. However, the current formulation of the tablets makes it difficult to administer to younger children, and this means that some 50 million children go untreated every year.
The Pediatric Praziquantel Consortium, with WHO assistance, is aiming to develop a new formulation of praziquantel that will be suitable for children aged 3 months to 5 years.
This could answer the overwhelming demand for treatment in this younger age group as well as enable the integration of this age group with regular preventive chemotherapy campaigns.
R&D blueprint
The road map for 2021–2030 sets global targets and milestones to prevent, control, eliminate or eradicate the 20 diseases and disease groups known collectively as NTDs. It also sets cross-cutting targets aligned with WHO’s 13th General Programme of Work and the Sustainable Development Goals (SDGs), with strategies for achieving these targets during the next decade.
To reach these targets, research and innovation have a critical role to play.
The R&D Blueprint for NTDs will set out the research and development priorities for the global NTD community, to address the critical gaps that might otherwise prevent the 2030 targets from being achieved.
Those priorities will be reassessed every two years to ensure that countries, funders, and researchers are prioritising the areas which will have the most impact.
Countries are both the drivers and the beneficiaries of progress towards the 2030 targets for NTDs. National and local governments must therefore play a central role in defining the research agenda. Multisectoral action must be fostered proactively both at the ground and ministerial levels if research is to be effectively translated into policy.
