Essential Medicines |
Achieving universal health coverage requires access to safe, effective, quality and affordable essential medicines, vaccines and health products.
Essential medicines are those that satisfy the priority health care needs of the population. They are intended to be available within the context of functioning health systems at all times, in adequate amounts, in appropriate dosage forms, of assured quality, and at prices that are affordable for patients and health systems alike. Selection of a limited number of essential medicines, taking into consideration national disease burden and clinical need, can lead to improved access through streamlined procurement and distribution of quality-assured medicines, support appropriate prescribing and use, and lower costs both for patients and healthcare systems.
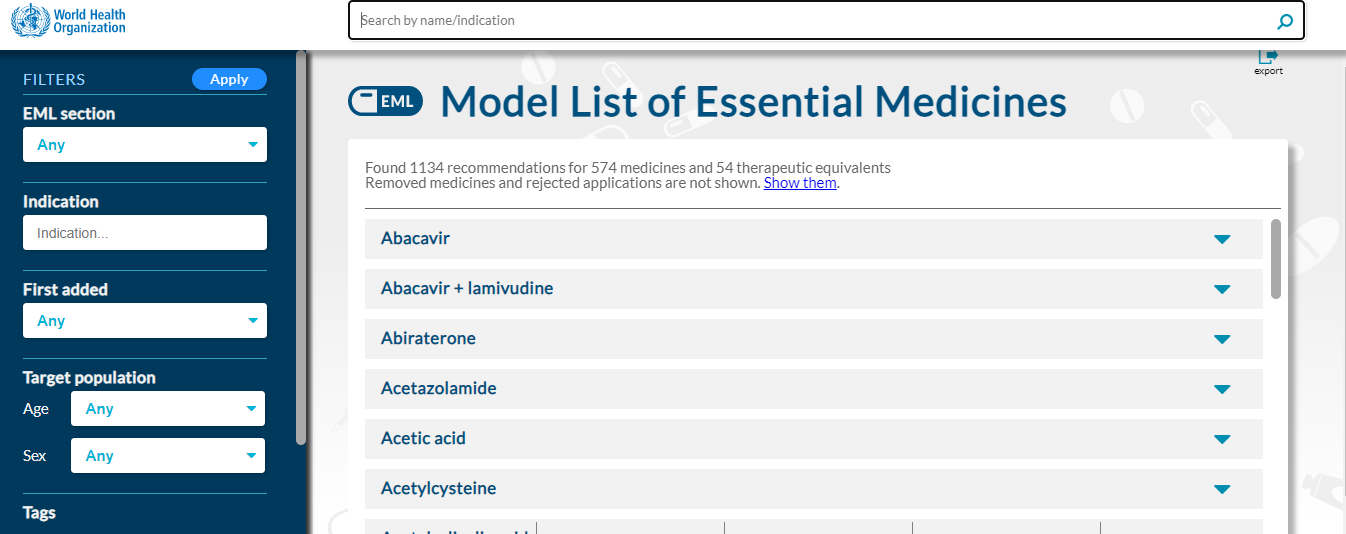
The WHO Model Lists of Essential Medicines (EML) and Essential Medicines for Children (EMLc) are key evidence-based guidance tools intended as a guide for countries or regional authorities to adopt or adapt in accordance with local priorities and treatment guidelines for the development of national essential medicines lists. They serve to help countries prioritize the selection, use and procurement of essential medicines, vaccines and health products, and inform national health policies, reimbursement decisions and access strategies.
The WHO Essential Medicines Lists are updated every two years following a rigorous, transparent, and evidence-based process. The WHO Expert Committee on the Selection and Use of Essential Medicines is responsible for providing recommendations to WHO regarding medicines on the WHO Essential Medicines Lists. Members of the Expert Committee are selected from WHO Expert Advisory Panels and are appointed by the WHO Director General based on their professional expertise and experience, while also ensuring geographical and gender balance in order to provide representation and practical experience from all regions of the world and across settings of all income levels.
The Expert Committee meets every two years to review applications for the addition, amendment or deletion of medicines on the Model Lists, taking into consideration the disease prevalence and public health relevance, scientific evidence of efficacy and safety, comparative costs, cost-effectiveness, and affordability, health system factors and availability. Notably, the absolute cost of a medicine is not a reason for the Expert Committee to not recommend a medicine be included on the Model Lists if it otherwise meets the required selection criteria.
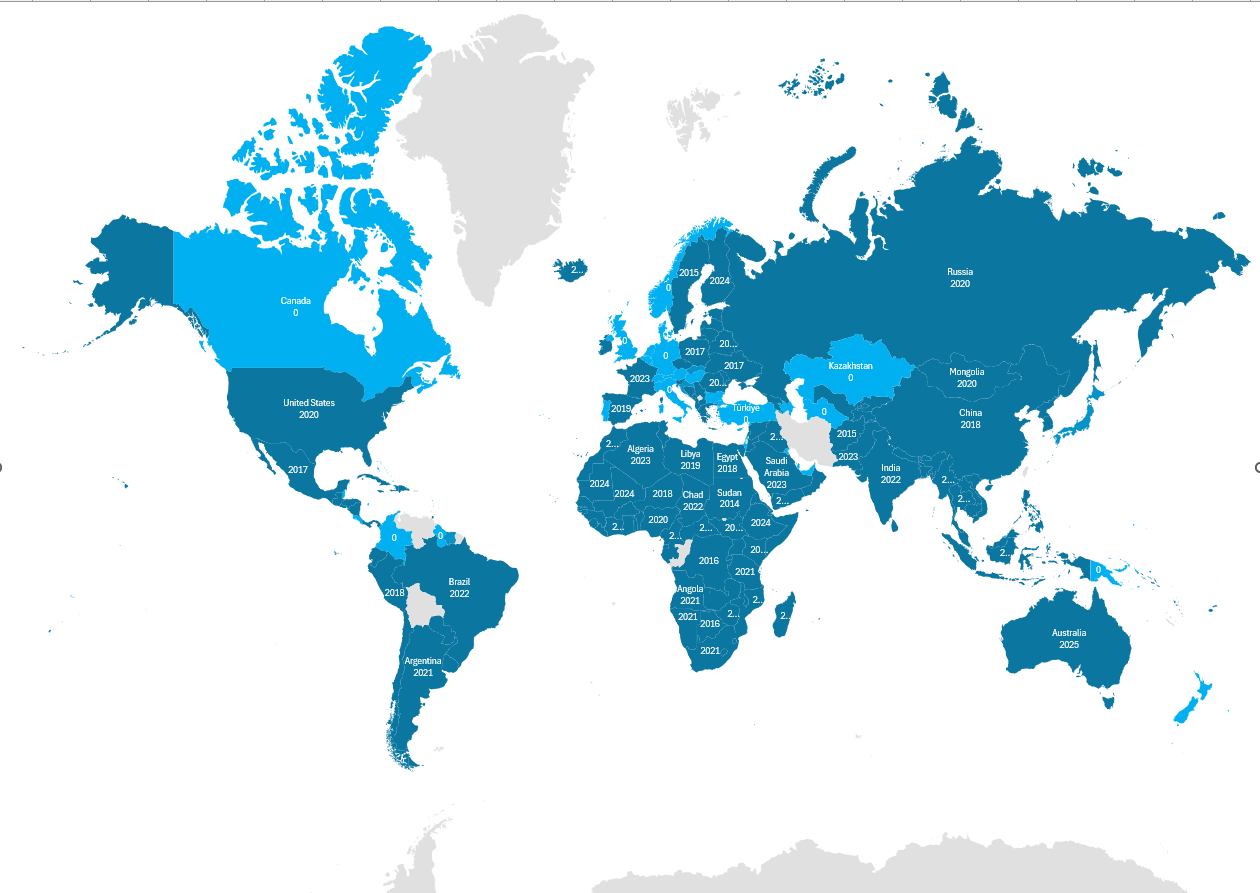
The WHO Essential Medicines Lists have been widely adopted globally, with many countries using them as a basis for their national essential medicines' lists. Over the past 25 years, more than 150 countries have developed and updated national essential medicines lists [1]. These lists influence medicine procurement decisions, supply chain management, and healthcare budgeting in their respective countries.
WHO’s work on improving access to essential medicines focuses on ensuring the availability of evidence-based guidance to support countries in the selection, use and accessibility of medicines to meet local, regional and national health priorities at all levels of the healthcare system. It has strong integration with other health system components such as treatment guidelines and reimbursement/insurance schemes. WHO’s work on essential medicines also recognizes the importance of regular updates to respond to changing health priorities and therapeutic advances.
Our Team |
Our Work |
Expert Commitee |
National EMLs |
Digital Library |
News
Publications
All →

The 24th meeting of the WHO Expert Committee on Selection and Use of Essential Medicines was held in Geneva, Switzerland, from 24 to 28 April 2023. The...

Information for applicants preparing a submission for the 2023 meeting of the WHO Expert Committee on...
The 24th meeting of the WHO Expert Committee on the Selection and Use of Essential Medicines to revise and update the WHO Model List of Essential Medicines...